
Impact Story: Developing the Tools to Evaluate Complex Drug Products: Peptides

As a class of drugs, peptides are increasingly important in medicine. FDA is developing the scientific tools to facilitate evaluations of these drug products and proposed generic equivalents.
Example of a Naturally Occurring Peptide: Bradykinin What are peptides? Peptides are sequences of molecules called amino acids. There are many varieties of amino acids, including 20 naturally occurring building blocks of proteins. Peptides of precise sequences may occur naturally in the body, but they may also be produced synthetically or using recombinant DNA technology in bacteria and other living systems. With their highly diverse sequence-dependent shapes and chemical and biological properties, these molecules are used to treat a variety of diseases, and applications for new peptide drug products are increasing. The naturally occurring peptide bradykinin, an important regulator of blood pressure, is a sequence of nine amino acids, each indicated above by a single letter. (The image of bradykinin shown was kindly made available by F.Vasconcellos based on sequence information in the PubCHEM database.)
What are peptides? Peptides are sequences of molecules called amino acids. There are many varieties of amino acids, including 20 naturally occurring building blocks of proteins. Peptides of precise sequences may occur naturally in the body, but they may also be produced synthetically or using recombinant DNA technology in bacteria and other living systems. With their highly diverse sequence-dependent shapes and chemical and biological properties, these molecules are used to treat a variety of diseases, and applications for new peptide drug products are increasing. The naturally occurring peptide bradykinin, an important regulator of blood pressure, is a sequence of nine amino acids, each indicated above by a single letter. (The image of bradykinin shown was kindly made available by F.Vasconcellos based on sequence information in the PubCHEM database.)
 What are peptides? Peptides are sequences of molecules called amino acids. There are many varieties of amino acids, including 20 naturally occurring building blocks of proteins. Peptides of precise sequences may occur naturally in the body, but they may also be produced synthetically or using recombinant DNA technology in bacteria and other living systems. With their highly diverse sequence-dependent shapes and chemical and biological properties, these molecules are used to treat a variety of diseases, and applications for new peptide drug products are increasing. The naturally occurring peptide bradykinin, an important regulator of blood pressure, is a sequence of nine amino acids, each indicated above by a single letter. (The image of bradykinin shown was kindly made available by F.Vasconcellos based on sequence information in the PubCHEM database.)
What are peptides? Peptides are sequences of molecules called amino acids. There are many varieties of amino acids, including 20 naturally occurring building blocks of proteins. Peptides of precise sequences may occur naturally in the body, but they may also be produced synthetically or using recombinant DNA technology in bacteria and other living systems. With their highly diverse sequence-dependent shapes and chemical and biological properties, these molecules are used to treat a variety of diseases, and applications for new peptide drug products are increasing. The naturally occurring peptide bradykinin, an important regulator of blood pressure, is a sequence of nine amino acids, each indicated above by a single letter. (The image of bradykinin shown was kindly made available by F.Vasconcellos based on sequence information in the PubCHEM database.)FDA considers any polymer composed of 40 or fewer amino acids to be a peptide.
Peptides can occur naturally in the body or can be produced in a laboratory through chemical synthesis or recombinant DNA technology using other living systems (e.g., bacteria). For example, bradykinin (Image 1) is a peptide that is a naturally occurring hormone in the body and controls blood pressure while glatiramer acetate is a peptide drug product that is created in a laboratory and intended to treat multiple sclerosis. Peptide drug products play a significant role in providing necessary medications for the public, but the manufacture of peptide drug products presents unique challenges. Moreover, the manufacturing of generic peptide drug products that are equivalent to their brand name counterparts has been particularly challenging. Currently, there are approximately 100 peptide drug products marketed in the U.S., Europe, and Japan. Annual global sales for peptide drug products are estimated to be $15 billion to $20 billion.
The Scientific Challenge for Approval of Generic Peptide Drug Products
FDA has seen a rapid increase in the number of new drug applications submitted for peptide drug products. The availability of generic versions of these products will be critical to increasing public access to these important medications. However, ensuring the quality and equivalence between generic and brand name peptide drug products raises a number of challenges, and those challenges differ according to the type of peptide drug.
For peptide drug products with a specifically defined sequence of amino acids, the challenge has been with impurities that may be inadvertently introduced during the production process that may affect a proposed generic drug’s safety profile. Peptide-related impurities can be especially difficult to detect, analyze, and control because they usually have similar sequences to the drug itself.
For peptide drug products with a specifically defined sequence of amino acids, the challenge has been with impurities that may be inadvertently introduced during the production process that may affect a proposed generic drug’s safety profile. Peptide-related impurities can be especially difficult to detect, analyze, and control because they usually have similar sequences to the drug itself.
For peptide drug products that vary in amino acid length and sequence, e.g., glatiramer acetate, the challenge has been with analyzing a proposed generic product to evaluate whether it has the same active ingredient as the brand name product. This type of analysis requires advanced analytical methods and novel statistical methods to determine pharmaceutical equivalence based on a large dataset of characterizations.
FDA’s Research to Support Development and Evaluation of Peptide Drug Products
Example of LC-HRMS analysis of a peptide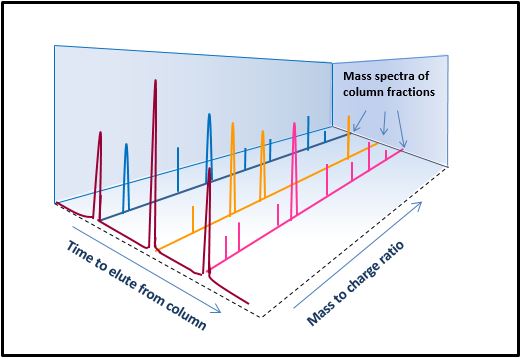 Developing a method to evaluate peptide products. In liquid chromatography-high resolution mass spectrometry (LC-HRMS), complex mixtures, such as peptide products and their impurities, are first passed through a column containing an adsorbent material through which liquid is flowing. Because components of the complex mixture interact differently with the material in the LC-HRMS, they are separated during their flow through the column, as shown in the tracing labeled as “Time to elute from column”. After being passed through the column, individual fractions of the collected liquid are analyzed by a mass spectrometry (MS) detector. In MS, electrical charges are added to the molecules in column fractions, and the molecules are then separated by an electrical field according to their mass-to-charge ratio. An individual peak on the three long axes shown in this image represents detected molecular components or its fragments. CDER researchers have shown that LC-HRMS can precisely identify the many types of molecules in complex peptide mixtures and their amounts. The sensitivity and precision of the technique and the huge amount of information it can provide on the constituents of complex products make it a valuable tool for comparing generic and brand name versions of peptide products (and many other complex products).
Developing a method to evaluate peptide products. In liquid chromatography-high resolution mass spectrometry (LC-HRMS), complex mixtures, such as peptide products and their impurities, are first passed through a column containing an adsorbent material through which liquid is flowing. Because components of the complex mixture interact differently with the material in the LC-HRMS, they are separated during their flow through the column, as shown in the tracing labeled as “Time to elute from column”. After being passed through the column, individual fractions of the collected liquid are analyzed by a mass spectrometry (MS) detector. In MS, electrical charges are added to the molecules in column fractions, and the molecules are then separated by an electrical field according to their mass-to-charge ratio. An individual peak on the three long axes shown in this image represents detected molecular components or its fragments. CDER researchers have shown that LC-HRMS can precisely identify the many types of molecules in complex peptide mixtures and their amounts. The sensitivity and precision of the technique and the huge amount of information it can provide on the constituents of complex products make it a valuable tool for comparing generic and brand name versions of peptide products (and many other complex products).
 Developing a method to evaluate peptide products. In liquid chromatography-high resolution mass spectrometry (LC-HRMS), complex mixtures, such as peptide products and their impurities, are first passed through a column containing an adsorbent material through which liquid is flowing. Because components of the complex mixture interact differently with the material in the LC-HRMS, they are separated during their flow through the column, as shown in the tracing labeled as “Time to elute from column”. After being passed through the column, individual fractions of the collected liquid are analyzed by a mass spectrometry (MS) detector. In MS, electrical charges are added to the molecules in column fractions, and the molecules are then separated by an electrical field according to their mass-to-charge ratio. An individual peak on the three long axes shown in this image represents detected molecular components or its fragments. CDER researchers have shown that LC-HRMS can precisely identify the many types of molecules in complex peptide mixtures and their amounts. The sensitivity and precision of the technique and the huge amount of information it can provide on the constituents of complex products make it a valuable tool for comparing generic and brand name versions of peptide products (and many other complex products).
Developing a method to evaluate peptide products. In liquid chromatography-high resolution mass spectrometry (LC-HRMS), complex mixtures, such as peptide products and their impurities, are first passed through a column containing an adsorbent material through which liquid is flowing. Because components of the complex mixture interact differently with the material in the LC-HRMS, they are separated during their flow through the column, as shown in the tracing labeled as “Time to elute from column”. After being passed through the column, individual fractions of the collected liquid are analyzed by a mass spectrometry (MS) detector. In MS, electrical charges are added to the molecules in column fractions, and the molecules are then separated by an electrical field according to their mass-to-charge ratio. An individual peak on the three long axes shown in this image represents detected molecular components or its fragments. CDER researchers have shown that LC-HRMS can precisely identify the many types of molecules in complex peptide mixtures and their amounts. The sensitivity and precision of the technique and the huge amount of information it can provide on the constituents of complex products make it a valuable tool for comparing generic and brand name versions of peptide products (and many other complex products).CDER researchers are evaluating a variety of sophisticated methods to determine how well each method can characterize peptides. Different types of techniques are becoming available that can be used independently or in conjunction with each other to gather comprehensive information on impurities, amino acid sequences, and distributions of peptide properties.
One powerful technique for analyzing peptides is mass spectrometry (MS).
In MS, charges are added to the various molecules in a drug product and the molecules are then exposed to the forces of an electrical field. The subsequent motion of these molecules depends on the ratio of their charge to their mass, making it possible to identify the individual molecular species among hundreds of components in a sample. Separating the components of a mixture using liquid chromatography (LC) prior to MS can achieve even better detection of the individual molecular components. Because of its high sensitivity, precision, and ability to separate peptides with similar sequences, this combination of analytical methods can be invaluable in detecting and analyzing individual components in a peptide drug product, which can be a peptide-related impurity in a single peptide drug or a particular sequence in a peptide mixture.
In the case of glatiramer acetate, CDER researchers applied a combination of different analytical methods that included nuclear magnetic resonance (NMR), asymmetric field flow fractionation (AFFF) coupled with multi-angle light scattering (MALS), and liquid chromatography coupled with mass spectrometry (LC-MS). The combination of these techniques helped to identify subtle differences, such as distributions of molecular weight, the amino acid compositions, and peptide sequence variations in the brand name drug product. This research informed FDA’s product-specific guidance on glatiramer acetate and the approval of the first generic glatiramer acetate in 2015.
Read a scientific article describing the use of these methods to compare related peptide products.
Read a scientific article describing the use of these methods to compare related peptide products.
How does this research advance the development of generic versions of peptide products?
The availability of highly sensitive and precise methods facilitates the characterization of complex generic and brand-name drug products. The results of this research assist drug developers who want to develop and market generic versions of peptide products and ultimately serve the American public by increasing access to these important medications.
Learn more about Generic Drugs Program
Select Publications
Zeng, Kui, et al. Liquid chromatography-high resolution mass spectrometry for peptide drug quality control. The AAPS Journal 17.3 (2015): 643–651.
Rogstad, Sarah, et al. Modern analytics for synthetically derived complex drug substances: NMR, AFFF–MALS, and MS tests for glatiramer acetate. Analytical and Bioanalytical Chemistry 407.29 (2015): 8647–8659.
Rogstad, Sarah, et al. Modern analytics for synthetically derived complex drug substances: NMR, AFFF–MALS, and MS tests for glatiramer acetate. Analytical and Bioanalytical Chemistry 407.29 (2015): 8647–8659.
Related Guidance
FDA distinguishes between peptides and proteins for regulatory purposes in the guidance for industry Biosimilars: Questions and Answers Regarding Implementation of theBiologics Price Competition andInnovation Act of 2009.





















.png)









No hay comentarios:
Publicar un comentario