
DoD, FDA working together to benefit warfighters

This is a depiction of the Department of Defense and the Food and Drug Administration logos
In a move to better serve the unique needs of the nation’s warfighters, leaders from the U.S. Food and Drug Administration and the Office of the Assistant Secretary of Defense for Health Affairs signed a memorandum of understanding to foster and prioritize the development of critical medical products.
The signing of the memorandum formalizes the partnership between the FDA and the Department of Defense that was authorized under a law enacted by Congress in 2017. Under this law, DoD is able to request help in speeding up development and review of products used to diagnose, treat, or prevent serious or life-threatening diseases and conditions faced by service members. It also allows for emergency use of medical products for threats that pose a specific risk to service members, including biological, chemical, radiological, or nuclear agents.
The FDA will continue to work with DoD to evaluate how to best access medical products that serve the military’s medical needs and rush review of priority DoD medical products. The FDA will also provide advice for the development and manufacturing of these products, and examine those that are already under development to determine which will streamline review.
The memorandum directs specific actions between the agencies, including semi-annual meetings at the senior-leader levels, quarterly meetings with FDA centers, and collaborations on emergency use of products.
Dr. Terry Rauch, acting deputy assistant secretary of Defense for Health Readiness Policy and Oversight, said the memorandum enables the Military Health System to speak through one voice with the FDA on strategic issues and to move forward on urgent needs with a prioritized DoD list for the FDA to focus on.
“DoD and FDA have always been committed to safe and effective products for our service members, and this [memorandum] really solidifies the needed relationship to provide these most effectively,” said Rauch. “We have already seen increased readiness due to the collaborative efforts with the approval of three medical products since the release of the law.”
The FDA has approved the use of an auto-injector for nerve agent exposure, a prophylactic drug for malaria, and the RECELL system for point-of-care skin regeneration in trauma patients. These products were approved after the release of the law, and the memorandum strengthens these efforts even further, he said.
“These products are critical to warfighter readiness in deployed environments and to the far-forward care settings that we face,” said Rauch. “Battlefield trauma and prolonged field care are at the forefront of challenges in military medicine.”
Earlier this year, the FDA granted emergency use authorization for DoD’s use of freeze-dried plasma. Rauch described it as a life-saving product designed for austere environments, which can now be used in a much more streamlined process under the authorization. In addition, the FDA approved a new malaria prophylactic drug, tafenoquine, in August.
“The FDA has been incredibly helpful and forward-leaning with all aspects of interaction on DoD medical product development,” said Rauch. “We are grateful that there is an increased interest by the leadership to drive collaboration, and we are leveraging that interest to the fullest.”
DoD Flu VE
Infographic
10/26/2018

Each season, several entities within the(DoD) perform surveillance for influenza among beneficiaries and utilize these data to perform VE analyses to estimate how well the seasonal vaccine protects against medically-attended influenza.
Psychiatric Medical Evaluations
Infographic
10/26/2018

This study evaluated incidence of pre-deployment family problem diagnoses and psychiatric medical evacuations among a population of active component service members without a history of previous mental health diagnoses, who deployed to the U.S. Central Command Area of Responsibility for the first time between 1 January 2002 and 31 December 2014.
Pelvic Inflammatory Disease
Infographic
10/26/2018

The purpose of this study was to update previous MSMR analyses of the incidence of acute Pelvic inflammatory disease (PID) among U.S. active component women using a 21-year surveillance period from 1996 through 2016. A secondary objective was to report on the proportion of service women with previously diagnosed PID who were subsequently diagnosed ...
USNS Comfort conducts mass casualty training exercise
Article
10/15/2018
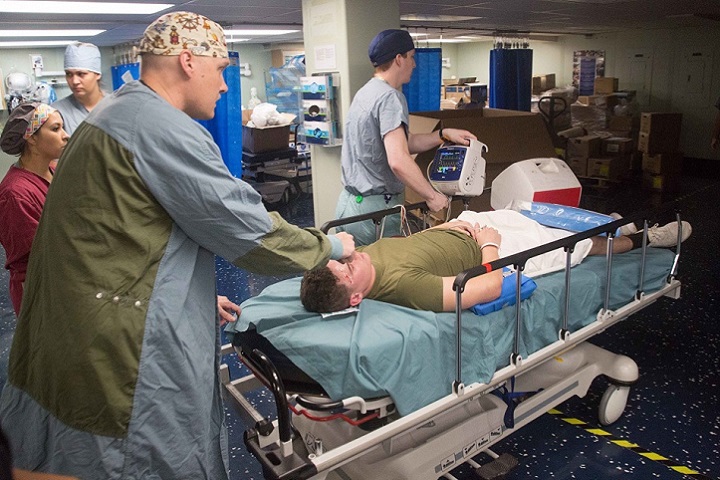
A mass casualty event, by nature, is chaotic
CVD
Infographic
10/3/2018

As of part of WOMEN’S HEALTH MONTH, we focus on the findings related to female service members. If the risk factors are recognized, these service members can take steps to modify their lifestyles or obtain appropriate medical intervention, and reduce the likelihood of significant CVD while serving in the Armed Forces, and also after leaving service.
Gynecologic Disorders
Infographic
10/3/2018

Gynecologic disorders are conditions that affect the female reproductive organs, including the uterus, ovaries, fallopian tubes, vagina, and vulva. As part of Women’s Health Month, this report describes the incidence and burden of four commonly occur-ring gynecologic disorders (menorrhagia, polycystic ovary syndrome (PCOS), uterine fibroids, and ...
HPV
Infographic
10/3/2018

Genital human papillomavirus (HPV) is the most common sexually transmitted infection (STI) in the U.S.; HPV is the second most frequently diagnosed STI in U.S. military service members. Currently, HPV vaccine is not mandatory for U.S. military service members, but the Defense Health Agency and each individual service have policies encouraging and ...
Say ‘Shoo’ to the flu with TRICARE
Article
9/26/2018
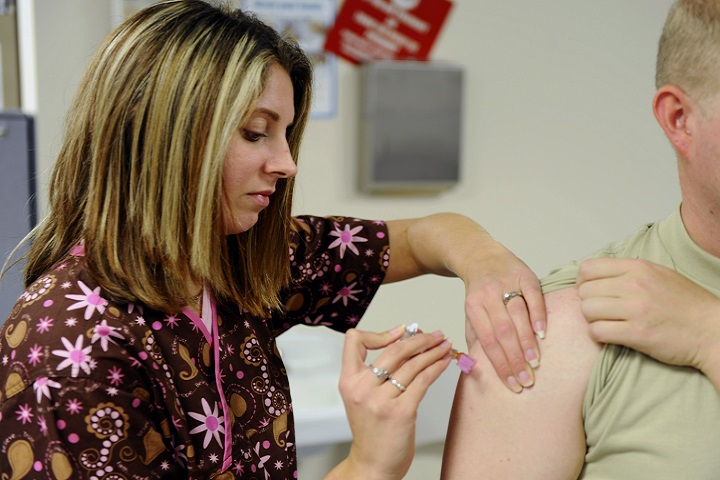
Flu viruses are serious, contagious viruses that can lead to hospitalization or even death
HPV
Infographic
9/24/2018

Genital human papillomavirus (HPV) is the most common sexually transmitted infection (STI) in the U.S., and is the second most frequently diagnosed STI in U.S. military service members. Currently, HPV is not a mandatory vaccine for U.S. military service. However, it is encouraged and offered to service members.
Drowning
Infographic
9/24/2018

Service members are at risk for unintentional drownings during training, occupational activities, and off-duty recreation. In the U.S., unintentional drowning ranks as the fifth leading cause of unintentional injury death and accounted for an average of 3,558 deaths (non-boating related) annually between 2007 and 2016. The current analysis extends ...
HIV
Infographic
9/24/2018

As part of the U.S. military’s total-force HIV screening program, civilian applicants for military service are screened for antibodies to HIV during pre-accession medical examinations. Infection with HIV is medically disqualifying for entry into U.S. military service. Since 1986, all members of the active and reserve components of the U.S. Armed ...
MHS Minute September 2018
Video
9/21/2018

Interested in hearing about some exciting events that took place around the Military Health System last month? Tune in to the MHS Minute to learn more!
Spine surgery team adds capability, improves readiness
Article
9/11/2018
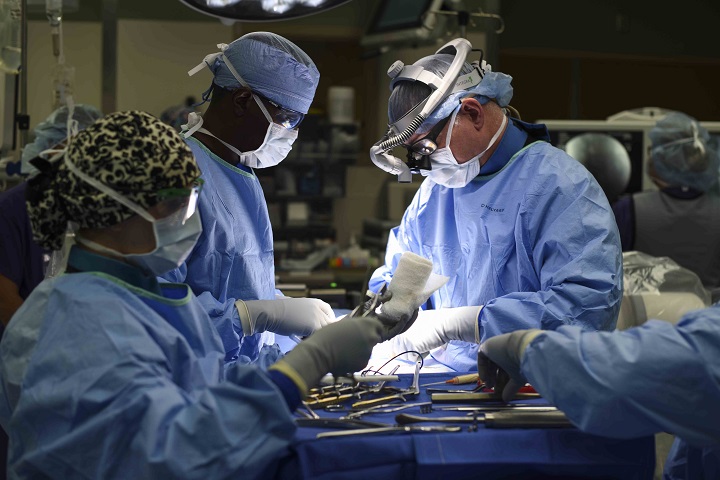
The benefits of performing complex surgeries in the orthopedic spine clinic go far beyond the operating room
Proper sleep hygiene as a force multiplier
Article
9/5/2018

Sleep is cheap; it costs nothing to rest troops properly, with proven, immediately realized returns
Army researchers develop tasty, healthy performance bar
Article
9/4/2018

Researchers aren’t working to provide recruits and soldiers with something that only tastes good; it has to make sense for their nutrition





















.png)









No hay comentarios:
Publicar un comentario