
Key Collaborative Factors When Medicaid Accountable Care Organizations Work With Primary Care Clinics to Improve Colorectal Cancer Screening: Relationships, Data, and Quality Improvement Infrastructure
ORIGINAL RESEARCH — Volume 16 — August 5, 2019
Melinda M. Davis, PhD1,2; Rose Gunn, MA1; Robyn Pham1; Amy Wiser, MD2; Kristen Hassmiller Lich, PhD3; Stephanie B. Wheeler, PhD, MPH3,4,5; Gloria D. Coronado, PhD6 (View author affiliations)
Suggested citation for this article: Davis MM, Gunn R, Pham R, Wiser A, Lich KH, Wheeler SB, et al. Key Collaborative Factors When Medicaid Accountable Care Organizations Work With Primary Care Clinics to Improve Colorectal Cancer Screening: Relationships, Data, and Quality Improvement Infrastructure. Prev Chronic Dis 2019;16:180395. DOI: http://dx.doi.org/10.5888/pcd16.180395.
PEER REVIEWED
On This Page
Summary
What is already known on this topic?
Partnering across organizational boundaries is critical to accountable care organization (ACO) success.
What is added by this report?
We explored how Oregon’s Medicaid ACOs are working with primary care clinics to improve the colorectal cancer (CRC) screening performance metric. We identified partnership, performance data, and quality improvement infrastructure as critical dimensions when ACOs and primary care clinics work to implement interventions to improve CRC screening. Unintended consequences included the potential exclusion of smaller clinics and metric focus and fatigue.
What are the implications for public health practice?
Practitioners looking to build cross-sector ACO–clinic partnerships to increase CRC screening or address other performance metrics should consider these 3 key collaborative factors and 2 unintended consequences.
Abstract
Purpose
Accountable Care Organizations (ACOs) are implementing interventions to achieve triple-aim objectives of improved quality and experience of care while maintaining costs. Partnering across organizational boundaries is perceived as critical to ACO success.
Methods
We conducted a comparative case study of 14 Medicaid ACOs in Oregon and their contracted primary care clinics using public performance data, key informant interviews, and consultation field notes. We focused on how ACOs work with clinics to improve colorectal cancer (CRC) screening — one incentivized performance metric.
Results
ACOs implemented a broad spectrum of multi-component interventions designed to increase CRC screening. The most common interventions focused on reducing structural barriers (n = 12 ACOs), delivering provider assessment and feedback (n = 11), and providing patient reminders (n = 7). ACOs developed their processes and infrastructure for working with clinics over time. Facilitators of successful collaboration included a history of and commitment to collaboration (partnership); the ability to provide accurate data to prioritize action and monitor improvement (performance data), and supporting clinics’ reflective learning through facilitation, learning collaboratives; and support of ACO as well as clinic-based staffing (quality improvement infrastructure). Two unintended consequences of ACO–clinic partnership emerged: potential exclusion of smaller clinics and metric focus and fatigue.
Conclusion
Our findings identified partnership, performance data, and quality improvement infrastructure as critical dimensions when Medicaid ACOs work with primary care to improve CRC screening. Findings may extend to other metric targets.
Introduction
Federal and state policies in the United States are increasingly moving away from pay for performance and toward reimbursement models that support the triple-aim objectives of improved quality and experience of care for populations while controlling costs (1). The Affordable Care Act, which was passed in March 2010, has many provisions that encourage achievement of the triple aim through expanded access to preventive care services, including encouraging cross-sector collaborations for care delivery through accountable care organizations (ACOs). ACOs are accountable for the quality and total costs of care for a defined population.
ACOs began in Medicare as a way to deliver high-quality, coordinated care; states have also expanded this model to Medicaid (2,3). Increased coordination and accountability in ACOs may lead to wiser spending and improved quality of care by delivering the right care to the right patient at the right time. In such cases, shared savings may be distributed across partner agencies (2). However, early research suggests there is considerable variation in partnership structures, decision making, and reimbursement models for ACOs (4,5). Moreover, the interventions that ACOs pursue and how they implement them may vary drastically and have implications for program effectiveness. Research suggests that ACO success will hinge on the ability of health care organizations to successfully partner across boundaries (6).
One quality indicator across many ACO and payer initiatives is colorectal cancer (CRC) screening (7,8). CRC is the second leading cause of cancer deaths in the United States, causing over 50,000 deaths annually (9). Guideline-concordant screening using endoscopic or fecal testing options can reduce CRC morbidity and mortality rates and is cost-effective (10,11). However, little research explores what interventions ACOs implement to increase CRC screening or how they work with primary care clinics.
Therefore, we sought to understand how ACOs work with primary care clinics to improve CRC screening. We focused on Oregon because of the opportunity to analyze 16 Medicaid ACOs (called coordinated care organizations or “CCOs”) to understand 1) which types of interventions CCOs are using to improve CRC screening rates and 2) how CCOs work with primary care clinics to implement the target interventions. Our study was designed to be hypothesis generating and to suggest promising practices to facilitate effective ACO–clinic partnerships to achieve performance benchmarks for CRC screening.
Methods
In 2011 the Oregon legislature passed House Bill 3650, authorizing the formation of CCOs. By statute, CCOs are governed by a partnership between those taking financial risk, professionals in the local health system (eg, doctors, hospitals), and community members; no CCO directly owns primary care clinics (12). CRC screening has been a CCO quality incentive metric since program inception, with annual reporting initiated in 2013.
We conducted a cross-case comparative study of CCOs in Oregon by using public performance data, transcripts from key informant interviews, and field notes from technical assistance consultations with CCO leadership. Our study was conducted in 2016, four years after CCO formation began. The institutional review board at Oregon Health and Science University approved this study (no. 11454).
Data collection and participant sampling
First, we collected publicly reported data about CCO characteristics and CRC screening performance in early 2016; we added 2016 CRC screening rates when they became available in 2017. Second, 2 members of the study team (M.M.D., R.P.) conducted CRC technical assistance consultation meetings with CCO leadership and quality improvement teams during June and July of 2016. Finally, one member of the study team (M.M.D.) conducted key informant interviews with a purposive sample of stakeholders from CCOs, primary care clinics, and the state from February 2016 through August 2016. Interviews followed a semistructured guide designed to clarify our understanding of how CCOs worked with clinics to address the CRC incentive metric. Interviews lasted approximately 60 minutes (range, 31–118 min) and were audio recorded and professionally transcribed.
Data management and analysis
Interview transcripts were checked for accuracy, and data were de-identified and analyzed using Atlas.ti version 7.0 (Atlas.ti Scientific Software). We found that existing conceptual frameworks and models did not account for the developmental nature of ACO and clinic partnerships over time (13). Therefore, we analyzed our data inductively to allow key themes to emerge naturally from the data.
We collected and analyzed data concurrently until saturation was reached (14). We used an iterative approach informed by Crabtree and Miller’s 5-stage immersion-crystallization analysis process (15). First, 2 authors (M.M.D., R.P.) reviewed transcripts and coded key segments of text with descriptive names (eg, partnership development, intervention targets) using a group process. Second, we reviewed data from a single CCO to understand how the organization was approaching CRC screening improvement and how they engaged primary care clinics and other stakeholders in this work. In a third cycle, 3 authors (M.M.D., R.P., R.G.) conducted a cross-case comparative analysis to identify patterns in CCO–clinic partnerships and associated performance on the CRC screening metric. We refined emerging themes with the larger study team and shared preliminary findings with OHA staff as a form of member checking (16). Use of reflexivity, multiple reviewers, data saturation, and an audit trail are associated with rigor in qualitative research methods (14,17).
Results
In 2015 the 16 CCOs ranged in size from 11,347 to 228,263 Medicaid enrollees and had an average CRC screening rate of 46.4% (Table 1). Qualitative data were gathered from 14 CCOs (88% response rate). Thirty-eight informants representing 10 CCOs participated in technical assistance consultations; 26 stakeholders representing 12 CCOs participated in key informant interviews. Interview participants represented CCO leadership (n = 16), primary care clinics (n = 6), and the state (n = 4).
Participating CCOs were actively implementing multiple intervention strategies, including those to increase community demand, increase community access, and increase provider delivery of CRC (Table 2). The most common intervention strategies were reducing structural barriers (85.7%, n = 12), delivering provider assessment and feedback (78.6%, n = 11), and offering patient reminders (50.0%, n = 7). All 14 CCOs implemented intervention strategies with sufficient evidence of effectiveness according to the Community Guide (www.thecommunityguide.org); more than half (n = 8) were also implementing interventions with insufficient evidence.
CCOs addressed 3 key areas when working with primary care clinics to improve CRC screening: 1) establishing relationships and building partnerships, 2) producing and sharing performance data, and 3) developing a process and infrastructure to support quality improvement (Figure). Illustrative quotes detailing these themes are in Table 3.
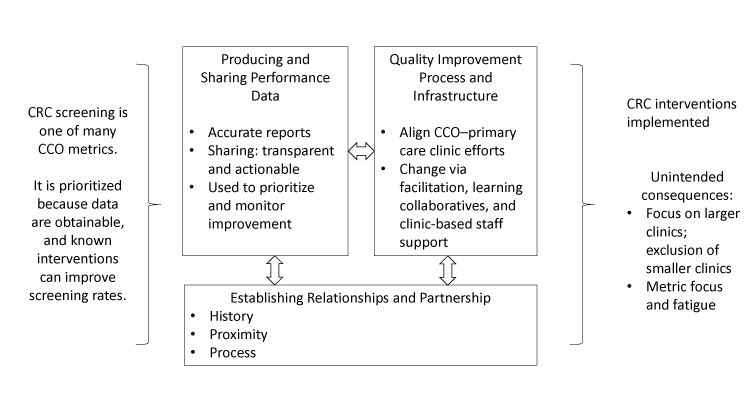
Figure.
Three key collaborative factors when Medicaid accountable care organizations work with primary care clinics to achieve performance metrics for CRC screening. Abbreviations: CRC, colorectal cancer; CCO, coordinated care organization. [A text description of this figure is available.]
Establishing relationships and building partnerships
Relationships played an important role in shaping how CCOs interacted with primary care clinics in their service region and in their ability to make improvements. Relationship quality could facilitate as well as impede the selection and implementation of interventions to increase CRC screening.
Prior history between CCO leadership and primary care stakeholders, physical proximity of the CCO’s infrastructure, and joint leadership roles in the CCO and regional clinics shaped the tenor of these relationships. One stakeholder noted, “We really just try to build the bond and leverage our existing relationships. . . . We had an advantage to be able to walk into the clinics and have a pretty long history of trust” (Participant 18). In contrast, CCOs that built on less-developed partnerships, strained relationships, or those that lacked a physical presence in the community faced challenges in raising local awareness and building trust.
CCOs developed or built on their relationships and partnerships with primary care clinics over time in 4 key ways. First, they had primary care providers and clinic leadership serve on the CCO board or on various subcommittees. Second, they hired local staff to provide ongoing support and to facilitate change in the primary care clinics. Third, CCO staff spent time listening, building trust, and aligning CCO initiatives with health system–level and clinic-level priorities and needs. Finally, CCOs created or expanded permanent physical space to house their staff in the local communities served.
Producing and sharing performance data
Performance data provided a starting point to prioritize and direct improvement activities for the CCOs and their contracted primary care clinics. CCOs used CRC screening data to inform targeted clinic outreach; motivate improvement at the clinic, provider, and team levels; and monitor progress toward performance goals. A first step was to obtain and share accurate performance data with clinics. One CCO medical director commented, “I think everyone assumes they’re doing a good job, until we can present them with credible evidence otherwise” (Participant 14). Some CCOs withheld incentive metric resources in early years to “put a system together to provide data to our partners so that they could do that improvement work on accurate, reliable data” (Participant 25).
CCO leadership anticipated that routinely sharing performance data and gap lists for CRC screening would enable clinics to “scrub their schedules as people are coming in or be reaching out to patients [using a population management approach]” (Participant 16). Over time, CCOs learned to be more strategic in how they distributed the gap lists for CRC screening — in terms of who at the clinic received them and how the data were presented and/or accessed — and they created processes to increase data accuracy by enabling clinics to amend CCO claims data with historical screening recorders. Low-quality, inaccurate data were poorly received by clinic partners. One clinic member said, “We would get reams of paper, and about the fourth or fifth page in when three-quarters [of the patients] . . . weren’t assigned to us we saw them as un-useful and put them aside” (Participant 8).
CCOs that had good standing relationships with clinics and the ability to generate metric data could also promote friendly, productive competition with transparent reporting of metric performance data, as illustrated in the following quote:
[Routine sharing of identified performance data] has generated competition, transparency, and a spirit of collaboration. Clinics can look at each other and say, “Boy, you’re doing great. Tell me what your secret is and let’s figure this out together, and will you help us? What did you do to get from here to here?” (Participant 12)
However, clinics varied in their ability to respond to performance data. Some clinics distributed performance data to panel managers who would then reach out to patients. Without dedicated staff to process or act on the CCO reports, the data languished at a clinic.
Developing a process and infrastructure to support quality improvement
Relationships and data allowed CCOs to partner with clinics and health system leadership to focus on quality improvement initiatives at the clinic level. CCO-funded regionally based improvement staff focused on building relationships and supporting clinics as they worked to achieve the incentive metrics, including CRC screening. One CCO improvement facilitator described how the metrics were straightforward to understand, but the approach to achieve these metrics at each clinic required targeted support. Facilitators described asking, “What does it take for this clinic to implement this?” (Participant 4), then building a tailored improvement approach that attended to clinic needs (eg, addressing leadership, understanding and using data, improving team functioning). Improvement facilitators often began by educating providers on the quality metrics then helping clinics refine clinical workflows or implement strategies to improve service delivery frequency.
Some CCOs also led regional learning collaboratives and funded clinic-based quality improvement staff. Learning collaboratives allowed quality improvement leads and staff from regional clinics to gather and share best practices, troubleshoot workflows, and plan their own initiatives. Clinic-based quality improvement staff helped lead clinic change or were panel managers who performed key tasks to support improvement efforts for CRC screening and other incentive metrics.
Promising practices
Despite heterogeneity in interventions implemented across CCOs, certain patterns stood out as promising in relation to CCO–clinic partnerships to improve CRC screening. Stakeholders noted how certain CCOs leveraged their relationships with partner clinics or funded staff to help implement changes in care delivery needed to achieve CRC screening metric benchmarks. The ability to provide accurate data to prioritize action and improvement monitoring was also critical. However, clinics also needed a process for acting on this information. Although some clinics had robust quality improvement infrastructure, others needed resources and training to be able to review data, select interventions, and implement changes. In contrast, some CCOs with lower levels of clinic engagement and data reporting or sharing capacity implemented CRC initiatives that circumvented clinics (eg, offering fecal tests for CRC screening directly to Medicaid enrollees). Although CCO-led interventions could increase CRC screening rates, informants indicated that this approach contributed to over-screening by duplicating clinic-level workflows, raised concerns about legal ramifications in relation to patient follow-up on abnormal results, and reduced the willingness of clinics and health systems to collaborate.
Unintended consequences
Collaboration between CCOs and clinics suggested 2 emerging and unintended consequences: 1) prioritizing larger clinics and excluding smaller clinics and 2) metric focus and fatigue. The ability to generate high-quality data and the need to build relationships and quality improvement infrastructure led many CCOs to focus their attention and resources on larger clinics. Stakeholders expressed concern that some of the smaller clinics — which may have more limited quality improvement capacity to begin with and are often found in rural areas where screening disparities exist — were not given data reports from the CCO or support with improvement. One stakeholder commented, “Sadly, I think if you look at the large clinics that are doing well . . . we consider[ed] that a win and we move[d] on. I would hate for someone to not be screened [for CRC] just because of the clinic they chose” (participant 22).
A second unintended consequence was a focus on the CCO metrics to the exclusion of other factors associated with quality of care and feelings of metric fatigue. Stakeholders commented on the number of metrics that clinics are responsible for, the burden of capturing and reporting data, and the pressure for continual improvement. “People are just exhausted. They come to the end of a metric year and . . . it’s like fighting with every ounce of energy you have to make sure that you’ve got enough people under your belt to hit a particular metric” (participant 12).
Discussion
Our study explored how Medicaid ACOs (CCOs in Oregon) work with primary care clinics to improve CRC screening. CCOs addressed 3 key collaborative factors: establishing relationships and building partnerships, producing and sharing performance data, and developing quality improvement processes and infrastructure. All CCOs were implementing multi-component interventions, some with sufficient evidence and others with insufficient evidence of effectiveness. Access to and knowledge of the performance metrics and an expectation that clinics would take action to increase CRC screening improvement was necessary but not sufficient. Robust relationships, high-quality actionable data, and helping clinics fund and figure out how to make improvements are promising practices associated with enhanced CCO–clinic collaboration to increase CRC screening.
Two unintended consequences emerged in our exploration of CCO–clinic partnerships that warrant additional attention. First, neglect or exclusion of smaller clinics may increase CRC screening disparities, and smaller clinics may experience more barriers to implementing change (18–20). Including smaller clinics is critical in supporting improved care, given that 78% of patients in the United States still receive care in clinics with 10 or fewer physicians (21). Second, metric focus and fatigue suggests the need to attend proactively to provider and staff burnout, to support team-based care models, and to stay cognizant of what “gets missed” as ACOs and CCOs focus on quality metrics at the potential expense of quality (22).
Our study contributes to a growing body of literature on effective practices for ACOs and to the broader literature on cross-sector partnerships and multi-level interventions using CRC as a case study. Findings encourage use of participatory approaches that attend to local context and needs (23,24) and support improvement as a dynamic process within a complex system using a “best processes” orientation (25).
Two areas warrant additional consideration. First, our findings highlight the opportunities and challenges of building cross-sector partnerships to implement interventions that increase CRC screening. Stakeholders described the importance of building trusting relationships and basic infrastructure as part of efforts to implement evidence-based interventions in routine care. Although ACOs may want to focus on specific interventions first, building basic improvement capacity can lay the foundation for successful implementation later. Second, although selecting an evidence-based intervention is a key component of improvement practice, determining how to support implementation is a critical determinant of intervention success. Facilitation — or providing support to aid implementation — is increasingly recognized as a critical factor of implementation success (26,27). Facilitators may engage key partners to implement needed change, to create a safe space for data sharing and reflection on improvement targets, and to optimize intervention delivery and understanding over time (28,29). Finally, our study findings suggest that in certain cases ACOs may also need to provide internal staffing support to enable clinics to implement interventions to achieve performance benchmarks. Even if well-intentioned, providing technical support without considering how to resource or to reward clinics and staff for making change may be poorly received and lack anticipated impact (30).
Our study has limitations. First, our data were cross-sectional. Although stakeholders described how CCOs were evolving their strategies over time, we were not able to evaluate these changes in detail or to definitively identify successful and unsuccessful intervention or implementation strategies. Future studies would benefit from assessing changes in CCO approaches over time, and their association with performance metrics. Also, we focused on how CCOs worked with primary care clinics on one metric, CRC screening. It is possible that different metrics may require other strategies to address. Regardless, our findings are likely generalizable to other preventive screenings.
Partnerships are perceived as critical to ACO success. We found that Oregon Medicaid ACOs engaged with primary care clinics to improve CRC screening by implementing multi-component interventions (eg, reducing structural barriers, delivering provider assessment and feedback, providing patient reminders). Facilitators of successful collaboration included a history of and a commitment to collaboration, the ability to provide accurate data to prioritize action and monitor improvement, and supporting clinics’ reflective learning through facilitation, learning collaboratives, and support of clinic-based staff. Perceived exclusion of smaller clinics and metric focus and fatigue emerged as unintended consequences of these improvement efforts and warrant additional attention. ACO–clinic partnerships must go beyond simply sharing what is needed for improvement to helping clinics figure out how to make improvements, which may include resourcing external and internal infrastructure. Our findings can inform ACOs how to effectively partner with primary care clinics to improve CRC screening and may extend to other performance metrics.
Acknowledgments
The authors appreciate the time and insight of the stakeholders who participated in this research. Copyrighted material was not used in this manuscript. This study was supported, in part, by cooperative agreement no. U48-DP005017 from the Centers for Disease Control and Prevention’s Prevention Research Centers Program and the National Cancer Institute (NCI) as part of the Cancer Prevention and Control Research Network and by funding opportunity no. CMS-1G1-12-001 from the US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Dr Davis was supported by an Agency for Healthcare Research and Quality patient centered outcomes research K12 award (award no. K12 HS022981 01) and an NCI K07 award (no. 1K07CA211971-01A1). The content provided is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Findings from this study were presented at the 2017 North American Primary Care Research Group Annual Meeting in Montreal, Canada.
Author Information
Corresponding Author: Melinda M. Davis, PhD, Department of Family Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd, Mail Code L222, Portland, OR 97239. Telephone: 503-494-4365. Email: davismel@ohsu.edu.
Author Affiliations: 1Oregon Rural Practice-based Research Network, Portland, Oregon. 2Department of Family Medicine, Oregon Health and Science University, Portland, Oregon. 3Department of Health Policy and Management, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. 4Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. 5Center for Health Promotion and Disease Prevention, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina. 6Center for Health Research, Kaiser Permanente, Portland, Oregon.
References
- Whittington JW, Nolan K, Lewis N, Torres T. Pursuing the triple aim: the first 7 years. Milbank Q 2015;93(2):263–300. CrossRefPubMed
- Centers for Medicare and Medicaid Services. Accountable Care Organizations (ACOs); 2017. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ACO/. Accessed April 13, 2018.
- Center for Health Care Strategies. Medicaid accountable care organizations: state update; 2018. https://www.chcs.org/resource/medicaid-accountable-care-organizations-state-update/. Accessed April 13, 2018.
- Trosman JR, Weldon CB, Douglas MP, Deverka PA, Watkins JB, Phillips KA. Decision making on medical innovations in a changing health care environment: insights from accountable care organizations and payers on personalized medicine and other technologies. Value Health 2017;20(1):40–6. CrossRef PubMed
- Barnes AJ, Unruh L, Chukmaitov A, van Ginneken E. Accountable care organizations in the USA: types, developments and challenges. Health Policy 2014;118(1):1–7. CrossRef PubMed
- Lewis VA, Tierney KI, Colla CH, Shortell SM. The new frontier of strategic alliances in health care: New partnerships under accountable care organizations. Soc Sci Med 2017;190:1–10. CrossRef PubMed
- Kaiser Family Foundation. Mapping Medicaid delivery system and payment reform; 2015. https://www.kff.org/interactive/delivery-system-and-payment-reform/. Accessed October 16, 2017.
- Wang H, Qiu F, Gregg A, Chen B, Kim J, Young L, et al. Barriers and facilitators of colorectal cancer screening for patients of rural accountable care organization clinics: a multilevel analysis. J Rural Health 2018;3(2):202–12. PubMed
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) program; 2017. https://seer.cancer.gov/. Accessed October 16, 2017.
- Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, et al. Screening for colorectal cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;315(23):2576–94. CrossRef PubMed
- Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer 2006;107(7):1624–33. CrossRef PubMed
- Stock R, Goldberg BW, editors. Health reform policy to practice: Oregon’s path to a sustainable health system: a study in innovation. San Diego (CA): Elsevier; 2017.
- Centers for Disease Control and Prevention. Colorectal Cancer Control Program’s simplified logic mode; 2017. https://www.cdc.gov/cancer/crccp/logic.htm. Accessed September 14, 2017.
- Cohen DJ, Crabtree BF. Evaluative criteria for qualitative research in health care: controversies and recommendations. Ann Fam Med 2008;6(4):331–9. CrossRef PubMed
- Borkan J. Immersion/crystallization. In: Crabtree BF, Miller WL, editors. Doing Qualitative Research. Thousand Oaks (CA): Sage Publications; 1999: 179–194.
- Thomas DR. Feedback from research participants: are member checks useful in qualitative research? Qual Res Psychol 2017;14(1):23–41. CrossRef
- Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res 1999;34(5 Pt 2):1189–208. PubMed
- Meyers D, Miller T, Genevro J, Zhan C, De La Mare J, Fournier A, et al. EvidenceNOW: balancing primary care implementation and implementation research. Ann Fam Med 2018;16(Suppl 1):S5–11. CrossRef PubMed
- Balasubramanian BA, Marino M, Cohen DJ, Ward RL, Preston A, Springer RJ, et al. Use of quality improvement strategies among small to medium-size us primary care practices. Ann Fam Med 2018;16(Suppl 1):S35–43. CrossRef PubMed
- Nutting PA, Crabtree BF, Miller WL, Stange KC, Stewart E, Jaén C. Transforming physician practices to patient-centered medical homes: lessons from the national demonstration project. Health Aff (Millwood) 2011;30(3):439–45. CrossRef PubMed
- Levine DM, Linder JA, Landon BE. Characteristics and disparities among primary care practices in the United States. J Gen Intern Med 2018;33(4):481–6. CrossRef PubMed
- Kim LY, Rose DE, Soban LM, Stockdale SE, Meredith LS, Edwards ST, et al. Primary care tasks associated with provider burnout: findings from a Veterans Health Administration survey. J Gen Intern Med 2018;33(1):50–6. CrossRef PubMed
- Ramanadhan S, Davis MM, Armstrong R, Baquero B, Ko LK, Leng JC, et al. Participatory implementation science to increase the impact of evidence-based cancer prevention and control. Cancer Causes Control 2018;29(3):363–9. CrossRef PubMed
- Wheeler SB, Davis MM. “Taking the bull by the horns”: four principles to align public health, primary care, and community efforts to improve rural cancer control. J Rural Health 2017;33(4):345–9. CrossRef PubMed
- Trickett EJ, Beehler S, Deutsch C, Green LW, Hawe P, McLeroy K, et al. Advancing the science of community-level interventions. Am J Public Health 2011;101(8):1410–9. CrossRef PubMed
- Harvey G, Kitson A. Implementing evidence-based practice in healthcare: a facilitation guide. New York (NY): Routledge; 2015.
- Harvey G, Kitson A. PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci 2015;11(1):33. CrossRef PubMed
- Jordan ME, Lanham HJ, Crabtree BF, Nutting PA, Miller WL, Stange KC, et al. The role of conversation in health care interventions: enabling sensemaking and learning. Implement Sci 2009;4(1):15. CrossRef PubMed
- Edmondson AC. The three pillars of a teaming culture. Cambrdige (MA): Harvard Business Review; 2013.
- Casalino LP. Technical assistance for primary care practice transformation: free help to perform unpaid labor? Ann Fam Med 2018;16(Suppl 1):S12–5. CrossRef PubMed





















.png)









No hay comentarios:
Publicar un comentario