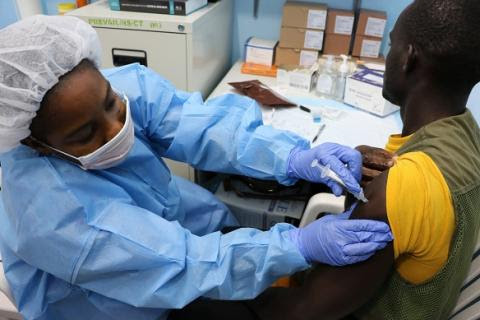
HHS Funds an Additional Year of Ebola Vaccine ManufacturingInvestigational Vaccine Proving to be Vital Tool in International Effort to Stop Second Deadliest Ebola Outbreak on RecordAs part of the international response to the Ebola virus outbreak in the Democratic Republic of the Congo (DRC) and to meet domestic biodefense goals, the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) will support manufacturing of an investigational Ebola vaccine, V920, from Merck & Co. Inc. of Kenilworth, New Jersey, known outside the U.S. and Canada as MSD. | 





















.png)









No hay comentarios:
Publicar un comentario