
Medical helps prevent the flu amongst service members through teamwork

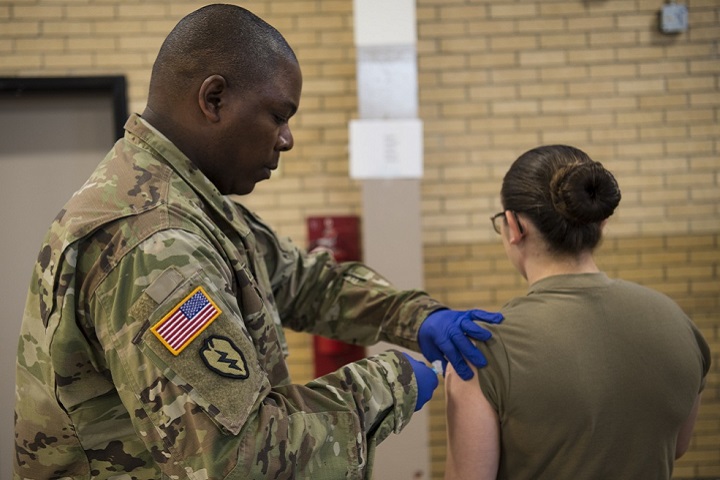
Members of DLA Troop Support Medical supply chain and DLA Distribution pose for a photo at an after action review for the Department of Defense’s Influenza Vaccination Program at Fort Detrick, Maryland Feb. 5, 2019. The Medical supply chain made approximately 3.4 million doses of the influenza vaccine available at military treatment facilities and U.S. Navy Fleet clinics around the world in support of the DOD’s Influenza Vaccination Program. (DoD photo by Alexander Quiñones)
PHILADELPHIA — If service members avoided the achy muscles, runny nose or fever symptoms that often come from the flu virus by getting a seasonal vaccination, they can thank the Defense Logistics Agency Troop Support for keeping them from getting sick.
The Medical supply chain made approximately 3.4 million doses of the influenza vaccine available at military treatment facilities and U.S. Navy Fleet clinics around the world in support of the Department of Defense’s Influenza Vaccination Program. For nearly a year, Medical worked with DLA Distribution and representatives from the Defense Health Agency and each military service to ensure program success during the 2018-2019 Influenza Season.
“The [DoD] Influenza Vaccine Program is an extremely complex operation, where no one component is more important than the other...without each partner in the process, it would never succeed,” said Dana Dallas, Medical cold chain program manager. “[All the partners] work extremely hard, over long days, overcoming every obstacle to ensure the warfighter and our treasured beneficiaries get the care they need...and we do a great job at it.”
Medical representatives, logistics counterparts, and DHA partners recently conducted an after action review at Fort Detrick, Maryland to discuss their efforts and results of the program. Medical credits the program’s success to teamwork.
“Every team member is dedicated to the success of the program and focused on meeting every deadline; ensuring a successful flu season,” said Theresa Rucci, Medical Pharmaceutical Distributor integrated support team chief.
The program’s goal was to have a 90 percent DoD compliant rate by Jan. 15. Medical and its partners were so successful that the DoD exceeded its goal with a 92 percent compliant rate, according to Rosalyn Brown, a DHA communications synchronization chief.
In addition to preventing the flu, the vaccine is viewed to be as important as other DoD protective items.
“The influenza vaccine is [one of the] ‘personal protective’ items issued to service members,” said Dr. Jay Montgomery, DHA’s North Atlantic Regional Vaccine Safety Hub Immunization Healthcare Branch Medical director. “Employed with other hygiene measures, the military’s influenza vaccination program has demonstrably improved operational effectiveness of active duty personnel and decreased morbidity and mortality among the broader Department of Defense’s beneficiary population.”
The annual program relies on Medical working with its partners to coordinate delivery of the vaccines. Medical is responsible for contracting, which includes awarding contracts and placing delivery orders, in addition to cold chain management, which includes providing technical input for packaging, tracking shipments and coordinating with customers.
Every January, the Pharmaceutical Manufacturer and Distributor Division begins procuring the vaccines and becomes the main point of contact for the services and vendors, said Rucci. Once the contracts are awarded, the Supply Support Division creates the vaccines’ ordering information.
Because the vaccines have a sensitive shelf life, the contracts require staggered deliveries to the DLA Distribution Depot Susquehanna Pennsylvania throughout the flu season. Once the vendor starts delivering vaccines to the depot, communication and coordination becomes vital.
“After the contracts are awarded it is imperative that the teams work together to ensure the close coordination of the shipments,” said Dallas. “The [influenza] vaccine is extremely temperature sensitive and cold chain shipping protocols must be maintained. All members of the team communicate very well with each other; always sharing information, details about customers, shipments, deliveries etc.”
By August, the Collective Customer Facing Division works with the DDSP in distributing vaccines from the depot to the customers. The DLA Troop Support Business Process Support office also gets involved to help with delivery efficiency and effectiveness.
Depending on customers’ availability, the delivery process can run until March. However, once the next January comes around, most of the deliveries are complete and the program comes full circle as the procurement process starts all over again for the upcoming flu season.
Although the program was a success, there were some challenges.
During the 2018 flu season, the program lost more vaccines than it did in 2017, due to weather and mechanical refrigeration related issues according to Jenna Wesolowski, a Medical contracting officer. However, because the program’s planning incorporates the need for extra quantities, known as “reship doses,” service members were not negatively affected.
“The flu team in Medical, on both the supplier and customer operations sides, does an outstanding job supporting the warfighter’s annual requirements,” said Alexander Quiñones, Pharmaceutical Manufacturer and Distributor Division chief. “Each year, there are always new challenges that present themselves, but I am always confident that the team will overcome anything they face and provide the best possible outcome to our customers worldwide.”
Wesolowski said the success of the Influenza Vaccination Program would not be possible without the relationship that Medical has with DLA Distribution.
It is a shared sentiment.
“The relationship built between Troop Support and Distribution has always been based on dedication to mission success and mutual respect,” said Robert Garrettson, a DLA Distribution Shelf Life Special Commodities team lead. “The [cold chain management] teams rely on each other’s professional expertise to provide the highest levels of customer satisfaction. Beyond seamlessly executing the influenza vaccine mission annually, both [major subordinate commands] work to enhance the program in an effort to achieve cost savings and better service.”
Disclaimer: Re-published content may have been edited for length and clarity. Read original post.Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger — United States, 2019
Report
2/8/2019
The 2019 child and adolescent immunization schedule summarizes the recommendations of the Advisory Committee on Immunization Practices (ACIP), including several changes from the 2018 immunization schedule.
Troop Support senior leaders discuss medical support as facilities transition to DHA
Article
2/1/2019

DLA Troop Support’s mission is focused on sustaining warfighter readiness and lethality by proactive global logistics in peace and war
Military Health System, industry allies work together to improve health care technology
Article
1/29/2019

Air Force Maj. Gen. Lee Payne visits University of Missouri’s Tiger Institute for Health Innovation
Vaccination is the best defense against the flu
Article
12/10/2018
Vaccination is needed every year because the Influenza viruses change
DHA expanding support to combatant commands
Article
11/30/2018

DHA headquarters will have centralized functional capabilities to ensure standardization and ensure efficiencies
Enhancing Combat Readiness
Presentation
11/28/2018
Maj Gen Lee Payne, USAF, MC, CFS, Assistant Director-Combat Support, Defense Health Agency
Updated Framework for Development of Evidence-Based Recommendations by the Advisory Committee on Immunization Practices
Report
11/16/2018
In 2010, the Advisory Committee on Immunization Practices (ACIP) implemented the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for developing evidence-based recommendations. Since its February 2018 meeting, ACIP has incorporated the use of Evidence to Decision or Evidence to Recommendation (EtR) frameworks into GRADE methodology.
Global Routine Vaccination Coverage — 2017
Report
11/16/2018
Global coverage with vaccines to prevent diphtheria, tetanus, pertussis, polio, and measles has remained at 84%–85% since 2010. Prioritizing countries with the highest number of unvaccinated children to implement context-specific strategies has the potential to increase immunization coverage globally.
General Best Practice Guidelines for Immunization
Publication
10/30/2018
The Centers for Disease Control and Prevention (CDC) recommends routine vaccination to prevent 17 vaccine-preventable diseases that occur in infants, children, adolescents, or adults. This report provides information for clinicians and other health care providers about concerns that commonly arise when vaccinating persons of various ages.
Immunization Tool Kit 9th Edition
Publication
10/25/2018
A practical reference that facilitates and enhances the global delivery of quality immunization healthcare to Department of Defense (DoD) beneficiaries and employees. The Defense Health Agency Immunization Healthcare Branch (DHA-IHB) publishes the Immunization Tool Kit based on national recommendations, evidenced-based, peer-reviewed published medical ...
WRAIR begins Phase 1 clinical trial of Marburg vaccine
Article
10/19/2018
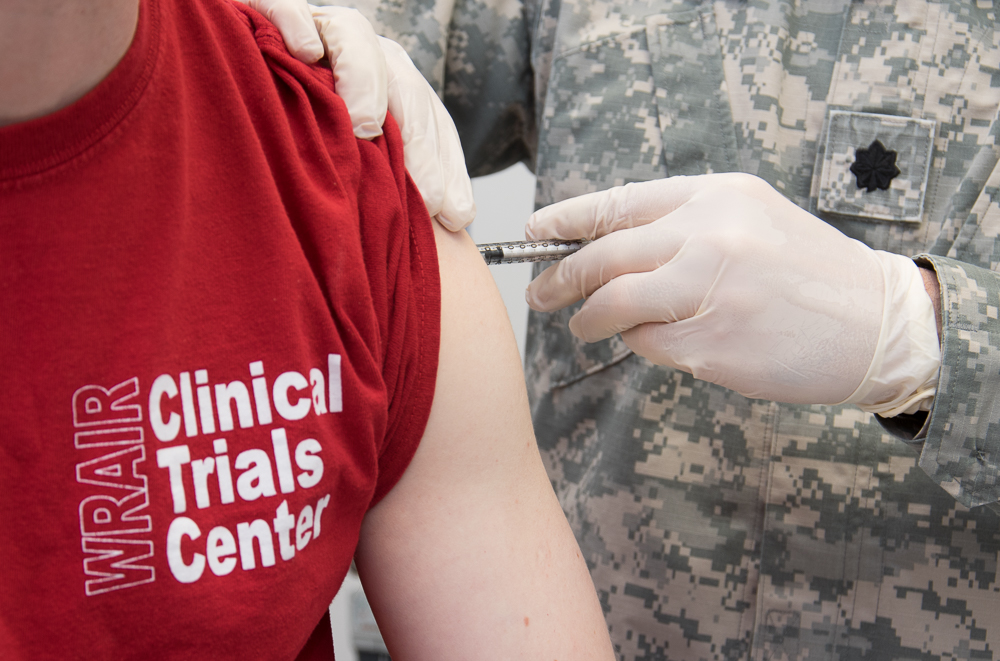
WRAIR recently administered the first vaccine in a Phase 1 clinical trial to evaluate the safety and immunogenicity of a Marburg vaccine
Vaccination Coverage for Selected Vaccines and Exemption Rates Among Children in Kindergarten — United States, 2017–18 School Year
Report
10/12/2018
Immunization programs conduct annual kindergarten vaccination assessments to monitor school-entry vaccination coverage for all state-required vaccines. Median vaccination coverage was 94.3% for 2 doses of measles, mumps, and rubella vaccine; 95.1% for the state-required number of doses of diphtheria and tetanus toxoids and acellular pertussis vaccine; and 93.8% for 2 doses of varicella vaccine.
Vaccination Coverage Among Children Aged 19–35 Months — United States, 2017
Report
10/12/2018
The Advisory Committee on Immunization Practices recommends routine vaccination by age 24 months against 14 potentially serious illnesses. In 2017, coverage with most recommended vaccines among children aged 19–35 months remained stable and high but was lower in more rural areas and among uninsured or Medicaid-insured children.
Say ‘Shoo’ to the flu with TRICARE
Article
9/26/2018
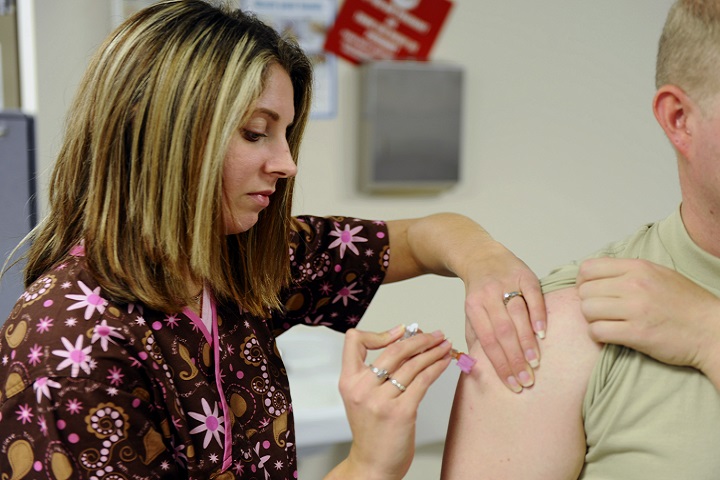
Flu viruses are serious, contagious viruses that can lead to hospitalization or even death
Trauma care reference body now woven into DHA combat support
Article
8/3/2018
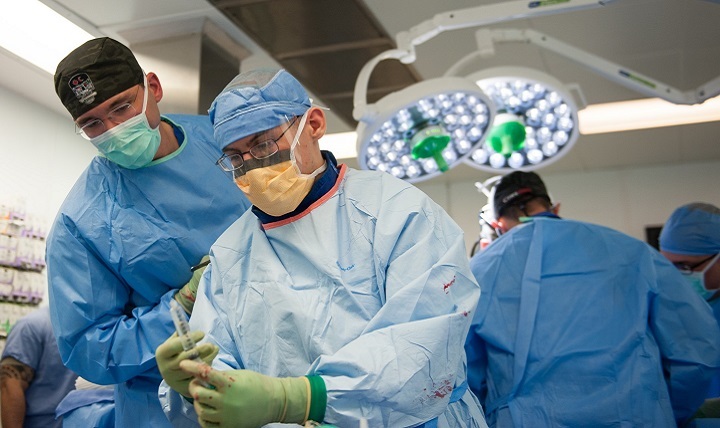
The JTS mission is to provide evidence-based process improvement of trauma and combat casualty care to drive morbidity and mortality to the lowest possible levels, and to provide recommendations on trauma care and trauma systems across the Military Health System





















.png)









No hay comentarios:
Publicar un comentario